How Many Grams Is 60 Ml
Greels
Mar 19, 2025 · 5 min read
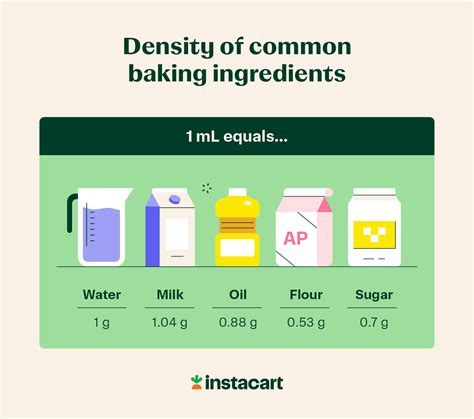
Table of Contents
How Many Grams is 60 ml? Understanding Volume, Weight, and Density
The question "How many grams is 60 ml?" doesn't have a single, simple answer. It's a common query, highlighting the crucial difference between volume (measured in milliliters, ml) and weight (measured in grams, g). To determine the weight, we need to know the density of the substance being measured. Density is the mass per unit volume, and it varies significantly between different materials.
This article will delve into the relationship between volume, weight, and density, explaining how to calculate the weight of 60 ml of various substances and offering practical examples. We'll also explore the importance of understanding these concepts in various fields and everyday life.
Understanding the Fundamentals: Volume, Weight, and Density
Before we tackle the conversion of 60 ml to grams, let's solidify our understanding of the key terms:
-
Volume: This refers to the amount of three-dimensional space occupied by a substance. Milliliters (ml) are a common unit for measuring volume, particularly for liquids. Think of it as how much space something takes up.
-
Weight: This is a measure of the force of gravity acting on an object's mass. Grams (g) are a unit of mass, often used interchangeably with weight in everyday contexts, particularly on Earth where gravity is relatively constant. Weight can change based on gravitational pull; your weight on the moon would be different than on Earth.
-
Density: This is the crucial link between volume and weight. Density is defined as the mass per unit volume. It's typically expressed in grams per milliliter (g/ml) or grams per cubic centimeter (g/cm³), where 1 ml is equivalent to 1 cm³. A high-density substance packs a lot of mass into a small volume, while a low-density substance has less mass in the same volume.
The Formula: The relationship between these three concepts can be expressed mathematically:
Density (g/ml) = Mass (g) / Volume (ml)
Therefore, to find the mass (in grams) you need to know the density and the volume:
Mass (g) = Density (g/ml) x Volume (ml)
Calculating the Weight of 60 ml of Different Substances
Now, let's apply this formula to calculate the weight of 60 ml for several common substances:
1. Water:
Water has a density of approximately 1 g/ml. This is why 1 ml of water is often considered to weigh approximately 1 gram.
Therefore, for 60 ml of water:
Mass (g) = 1 g/ml * 60 ml = 60 g
2. Milk:
Milk has a slightly higher density than water, typically around 1.03 g/ml.
Mass (g) = 1.03 g/ml * 60 ml = 61.8 g
3. Oil (Vegetable Oil):
Vegetable oil has a lower density than water, around 0.92 g/ml.
Mass (g) = 0.92 g/ml * 60 ml = 55.2 g
4. Mercury:
Mercury is a very dense liquid metal with a density of approximately 13.6 g/ml.
Mass (g) = 13.6 g/ml * 60 ml = 816 g
These examples highlight the significant difference in weight for the same volume (60 ml) depending on the density of the substance.
The Importance of Understanding Density
Understanding the concept of density is crucial in various fields and everyday applications:
-
Science and Engineering: Density calculations are fundamental in fields like chemistry, physics, and engineering. They're used in designing structures, calculating buoyancy, and understanding fluid dynamics.
-
Medicine: Density plays a role in medical diagnostics. For example, blood density can indicate certain health conditions.
-
Food Science: Density is crucial in food processing and packaging. Understanding the density of different ingredients is important for recipe formulation and determining the appropriate packaging size.
-
Environmental Science: Density is used to monitor water quality and assess pollution levels.
-
Everyday Life: Even in everyday life, an intuitive understanding of density helps us in various situations. For example, we instinctively know that a block of wood will float because it has a lower density than water.
Beyond Simple Calculations: Factors Affecting Density
While the calculations above provide a good starting point, it's important to acknowledge that density isn't always constant. Several factors can influence a substance's density:
-
Temperature: Most substances expand when heated, thus decreasing their density. This is why hot air rises.
-
Pressure: Increased pressure can compress a substance, increasing its density.
-
Concentration: For solutions and mixtures, the density depends on the concentration of the components. A sugary solution will be denser than pure water.
-
Phase Changes: A substance's density changes dramatically during phase transitions (solid to liquid, liquid to gas). Ice, for example, is less dense than liquid water.
Advanced Applications and Considerations: Specific Gravity
In many scientific and industrial contexts, specific gravity is a more practical measure than density. Specific gravity is the ratio of the density of a substance to the density of a reference substance, usually water at 4°C (its maximum density). This provides a dimensionless quantity, making comparisons easier.
Specific gravity doesn't require units, as it's a ratio. For example, a specific gravity of 1.03 for milk means its density is 1.03 times the density of water.
Conclusion: The Importance of Context
The question "How many grams is 60 ml?" highlights the crucial distinction between volume and weight. There is no single answer without knowing the substance's density. We've demonstrated how to calculate the weight given the density and explored the significance of density in various contexts. Remember to consider factors like temperature, pressure, and phase changes when dealing with density calculations in real-world scenarios. Understanding these fundamental concepts is essential for anyone working with materials, conducting experiments, or simply engaging in everyday problem-solving. The use of specific gravity offers a convenient, dimensionless measure for comparing densities of different materials. Ultimately, careful consideration of the material and its properties is key to accurately determining its mass based on a given volume.
Latest Posts
Latest Posts
-
What Is 5 Percent Of 20
Mar 19, 2025
-
How Many Miles In 200 Km
Mar 19, 2025
-
What Is 58 Cm In Inches
Mar 19, 2025
-
154 Cm To Feet And Inches
Mar 19, 2025
-
How Many Foot In 157 Cm
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Grams Is 60 Ml . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
