60 Grams Is How Many Ml
Greels
Mar 17, 2025 · 4 min read
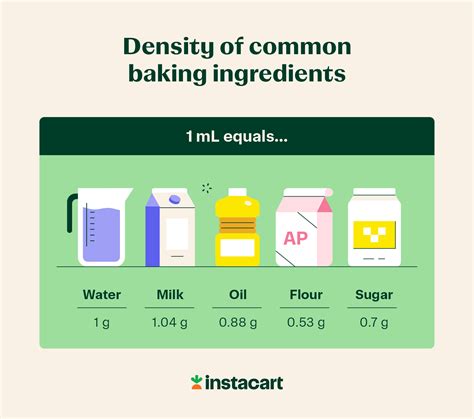
Table of Contents
60 Grams is How Many Milliliters? Understanding the Relationship Between Mass and Volume
The question, "60 grams is how many milliliters?" doesn't have a single, simple answer. This is because grams (g) measure mass, while milliliters (ml) measure volume. The conversion between the two depends entirely on the density of the substance being measured. Density is the mass per unit volume, often expressed as grams per milliliter (g/ml) or grams per cubic centimeter (g/cm³ – since 1 ml = 1 cm³).
To understand this crucial relationship, let's delve deeper into the concepts of mass, volume, and density. We'll then explore how to calculate the volume of different substances given their mass in grams. Finally, we'll address common misconceptions and provide practical examples to solidify your understanding.
Understanding Mass, Volume, and Density
-
Mass: Mass is the amount of matter in an object. It's a fundamental property and remains constant regardless of location or gravitational pull. We measure mass using units like grams, kilograms, and ounces.
-
Volume: Volume is the amount of space an object occupies. It's a three-dimensional measurement, and units include milliliters, liters, cubic centimeters, and cubic meters.
-
Density: Density is the relationship between mass and volume. It tells us how tightly packed the matter is within a given space. A high-density substance has a lot of mass packed into a small volume, while a low-density substance has less mass in the same volume. The formula for density is:
Density = Mass / Volume
Or, rearranged to find volume:
Volume = Mass / Density
This formula is the key to answering our original question. To convert 60 grams to milliliters, we need to know the density of the substance.
Calculating Volume from Mass: Examples
Let's explore several common substances and calculate their volume given a mass of 60 grams:
1. Water:
Water has a density of approximately 1 g/ml at 4°C (39.2°F). This means 1 gram of water occupies 1 milliliter of volume. Therefore, 60 grams of water occupies:
Volume = 60 g / 1 g/ml = 60 ml
So, 60 grams of water is equal to 60 milliliters.
2. Oil (Vegetable Oil):
Vegetable oil has a lower density than water, typically around 0.92 g/ml. To find the volume of 60 grams of vegetable oil:
Volume = 60 g / 0.92 g/ml ≈ 65.22 ml
Therefore, 60 grams of vegetable oil occupies approximately 65.22 milliliters.
3. Honey:
Honey is much denser than water, with a density ranging from 1.38 to 1.45 g/ml. Let's use the average of 1.415 g/ml:
Volume = 60 g / 1.415 g/ml ≈ 42.43 ml
60 grams of honey would occupy roughly 42.43 milliliters.
4. Mercury:
Mercury is extremely dense, with a density of approximately 13.5 g/ml. The volume of 60 grams of mercury would be:
Volume = 60 g / 13.5 g/ml ≈ 4.44 ml
This shows that a relatively small volume of mercury can have a significant mass.
5. Air:
Air's density is highly variable depending on temperature, pressure, and altitude. At standard temperature and pressure (STP), the density of air is approximately 1.225 kg/m³. To use this in our calculation, we need to convert to g/ml:
1.225 kg/m³ = 1.225 g/cm³ = 1.225 g/ml
Volume = 60 g / 1.225 g/ml ≈ 48.98 ml
This calculation shows the volume occupied by 60 grams of air under standard conditions.
The Importance of Density in Conversions
These examples clearly demonstrate that knowing the density of a substance is crucial for converting its mass to volume. Without knowing the density, you cannot accurately determine the volume. This is a fundamental principle in chemistry, physics, and other scientific fields.
Common Misconceptions
A frequent mistake is assuming that a direct conversion exists between grams and milliliters. There is no universal conversion factor because the relationship depends on density, which varies from substance to substance. Remember, grams measure mass, and milliliters measure volume. They are distinct concepts that are linked through density.
Practical Applications
Understanding the relationship between grams, milliliters, and density has several practical applications:
-
Cooking and Baking: Recipes often list ingredients by weight (grams) instead of volume (milliliters), particularly for dry ingredients. Knowing the density of ingredients can help you convert between measurements accurately.
-
Chemistry and Science: Accurate measurements of mass and volume are essential in various scientific experiments and calculations. Understanding density allows for precise conversions.
-
Engineering and Construction: Density plays a vital role in engineering calculations, especially in determining the weight and volume of materials used in construction projects.
-
Medicine and Pharmaceuticals: Accurate dosage is critical in medicine, and density is often considered when calculating and dispensing medications.
Conclusion
The question "60 grams is how many milliliters?" highlights the importance of understanding the relationship between mass, volume, and density. There is no single answer without knowing the density of the substance. This article has explored this relationship using examples, emphasizing the crucial role of density in conversion calculations. Remember, accurate measurements are essential in various fields, and understanding the concepts of mass, volume, and density is crucial for precise calculations and problem-solving. Always refer to reliable sources for the density of specific substances before performing conversions.
Latest Posts
Latest Posts
-
How Many Feet Is 300 Meters
Mar 17, 2025
-
How Many Inches Is 77 Cm
Mar 17, 2025
-
How Many Feet Are In 73 Inches
Mar 17, 2025
-
How Many Cm In 5 5 Inches
Mar 17, 2025
-
How Many Kg Is 210 Lbs
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about 60 Grams Is How Many Ml . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
