60 Grams Equals How Many Ml
Greels
Mar 17, 2025 · 5 min read
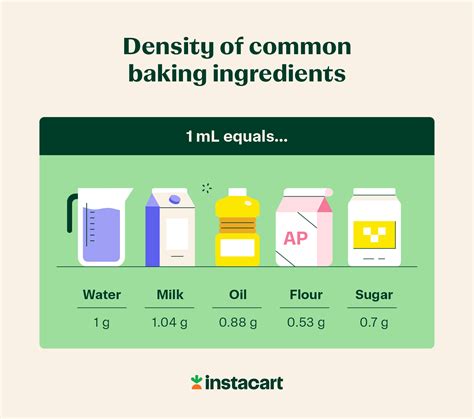
Table of Contents
60 Grams Equals How Many ml? Understanding Mass and Volume
The question "60 grams equals how many ml?" is a common one, but it doesn't have a single, simple answer. This is because grams (g) measure mass, while milliliters (ml) measure volume. The conversion depends entirely on the density of the substance being measured. Density is the mass per unit volume, often expressed as grams per milliliter (g/ml) or grams per cubic centimeter (g/cm³). Since 1 ml is equal to 1 cm³, these units are interchangeable in this context.
This article will explore this crucial concept, providing a clear understanding of how to convert grams to milliliters and addressing common misconceptions. We'll delve into different scenarios, practical examples, and helpful tips to ensure you can confidently perform these conversions in various situations.
Understanding the Relationship Between Mass, Volume, and Density
Before diving into the conversion, it's crucial to grasp the fundamental relationship between mass, volume, and density.
-
Mass: This refers to the amount of matter in an object. It's typically measured in grams (g), kilograms (kg), etc.
-
Volume: This refers to the amount of space an object occupies. It's commonly measured in milliliters (ml), liters (l), cubic centimeters (cm³), etc.
-
Density: This is the ratio of an object's mass to its volume. A denser object has more mass packed into a given volume. The formula for density is:
Density (ρ) = Mass (m) / Volume (V)
To convert grams to milliliters, you need to know the density of the substance. Once you have the density, you can rearrange the density formula to solve for volume:
Volume (V) = Mass (m) / Density (ρ)
Converting 60 Grams to Milliliters: Different Substances, Different Results
Let's illustrate this with examples. The conversion of 60 grams to milliliters will vary drastically depending on the substance:
1. Water:
Water has a density of approximately 1 g/ml at 4°C (39°F). This means 1 gram of water occupies 1 milliliter of volume. Therefore, 60 grams of water would occupy 60 milliliters. This is a convenient and often-used reference point.
2. Oil:
Vegetable oil, for instance, has a density slightly less than water, typically around 0.92 g/ml. To find the volume of 60 grams of oil, we use the formula:
V = m / ρ = 60 g / 0.92 g/ml ≈ 65.2 ml
Therefore, 60 grams of vegetable oil would occupy approximately 65.2 milliliters.
3. Mercury:
Mercury, a much denser liquid metal, has a density of about 13.6 g/ml. Using the formula:
V = m / ρ = 60 g / 13.6 g/ml ≈ 4.4 ml
Thus, 60 grams of mercury would occupy approximately 4.4 milliliters.
Common Substances and Their Densities: A Handy Reference
To successfully convert grams to milliliters, having a reference table of common substances and their densities is invaluable. Here's a partial list:
| Substance | Density (g/ml) |
|---|---|
| Water (4°C) | 1.00 |
| Vegetable Oil | ~0.92 |
| Ethanol (Alcohol) | ~0.79 |
| Mercury | ~13.6 |
| Gasoline | ~0.75 |
| Milk | ~1.03 |
| Honey | ~1.42 |
| Corn Syrup | ~1.38 |
| Aluminum (solid) | ~2.70 |
| Gold (solid) | ~19.3 |
This list demonstrates the significant differences in density between various substances. This difference highlights why a simple, universal conversion factor between grams and milliliters doesn't exist.
Practical Applications and Real-World Examples
Understanding the conversion between grams and milliliters has numerous practical applications across various fields:
-
Cooking and Baking: Recipes often list ingredients by weight (grams) rather than volume (milliliters). Knowing the density allows for accurate conversions, particularly for liquids like oil or honey, ensuring consistent results.
-
Chemistry and Science: In scientific experiments, precise measurements of mass and volume are crucial. Converting between these units is vital for accurate calculations and experimental reproducibility.
-
Medicine: Dosage calculations often involve conversions between mass and volume, especially when dealing with liquid medications.
-
Engineering and Manufacturing: Designing and manufacturing processes require accurate calculations involving mass and volume, impacting efficiency and product quality.
Beyond Liquids: Converting the Mass of Solids
While the examples above primarily focus on liquids, the principles apply to solids as well. However, the volume of a solid is often more complex to determine accurately. For regular-shaped solids (like cubes or spheres), the volume can be calculated using geometric formulas. For irregularly shaped solids, techniques like water displacement are used to determine the volume.
Potential Sources of Error and How to Minimize Them
Accuracy in converting grams to milliliters is critical. Several factors can introduce errors:
-
Temperature: Density changes with temperature. For instance, water's density is slightly less than 1 g/ml at room temperature.
-
Impurities: The presence of impurities in a substance can affect its density.
-
Measurement Errors: Inaccurate measurements of mass or volume will lead to errors in the calculated volume.
To minimize errors, use precise measuring instruments, control temperature, and ensure the substance is pure.
Conclusion: Mastering the Conversion of Grams to Milliliters
Converting 60 grams to milliliters isn't a simple matter of applying a single conversion factor. It requires understanding the fundamental relationship between mass, volume, and density, and knowing the density of the specific substance being measured. By mastering these concepts and using the formula V = m/ρ, you can confidently and accurately perform these conversions in various situations. Remember that accurate measurements are crucial for ensuring reliable results in all applications. Using a reference table of densities, practicing conversions, and being mindful of potential errors will build your proficiency and confidence in this essential skill.
Latest Posts
Latest Posts
-
How Much Is 165 Lbs In Kg
Mar 17, 2025
-
How Much Is 74 Kg In Lbs
Mar 17, 2025
-
How Many Pounds In 2 3 Kg
Mar 17, 2025
-
How Many Inches Is 85 Cm
Mar 17, 2025
-
How Many Oz Is 40 Grams
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about 60 Grams Equals How Many Ml . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
